Lambert-Eaton myasthenic syndrome (LEMS) is a rare autoimmune disease that affects approximately 1 in 100,000 people in the United States. The primary symptom is muscle weakness—the result of auto-antibodies to voltage-gated calcium channels, causing a reduction in the amount of acetylcholine (ACh) released from nerve terminals.1
The most common clinical presentations of LEMS are proximal muscle weakness and easy fatigability that may lead to difficulty walking and climbing stairs. The classical triad of LEMS includes proximal leg weakness, hyporeflexia or areflexia, and cholinergic dysautonomia (dry mouth, impotence, etc.) Ocular bulbar weakness is rare. Symptoms can be life threatening when the weakness involves respiratory muscles. Approximately 50%-60% of LEMS patients have an underlying malignancy, typically small cell lung cancer (SCLC).2-4
Amifampridine in LEMS
Catalyst has completed the FDA clinical development and secured FDA approval for amifampridine, a neuronal potassium channel blocker, for the treatment of LEMS.
Amifampridine previously received orphan drug designation and Breakthrough Therapy designation for LEMS from the FDA. In 2018, Catalyst filed an NDA for amifampridine use in LEMS. As of November 2018, amifampridine is the only evidence-based, FDA-approved treatment for adult patients with Lambert-Eaton Myasthenic Syndrome (LEMS).
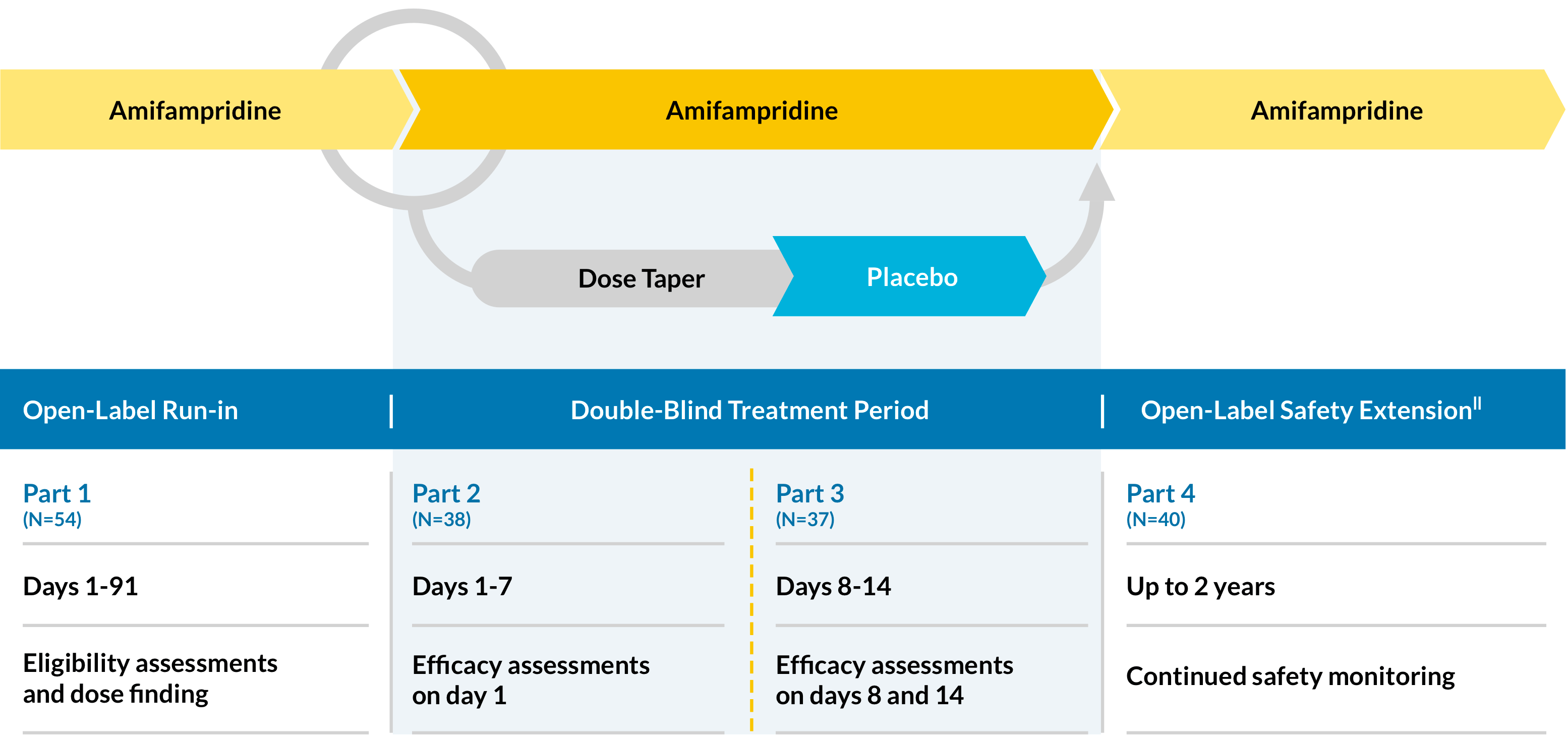
First LEMS Phase 3 Trial (LMS-002)
LMS-002 is a phase 3, multicenter, double-blind, placebo-controlled, randomized (1:1), discontinuation study, designed in 4 parts to evaluate the efficacy and safety of multiple-dose administration amifampridine in patients with LEMS.
Efficacy measures
Primary:
- Change in QMG score* from baseline (day 0) to day 14
- Change in SGI score† from baseline (day 0) to day 14
.
Secondary:
- Change in T25FW score‡ from baseline (day 0) to day 14
- Change in CGI-I score§ from baseline (day 0) to day 14
Safety assessments
Patient safety is continually assessed and dose adjustments are made during the 2-year open-label extension||
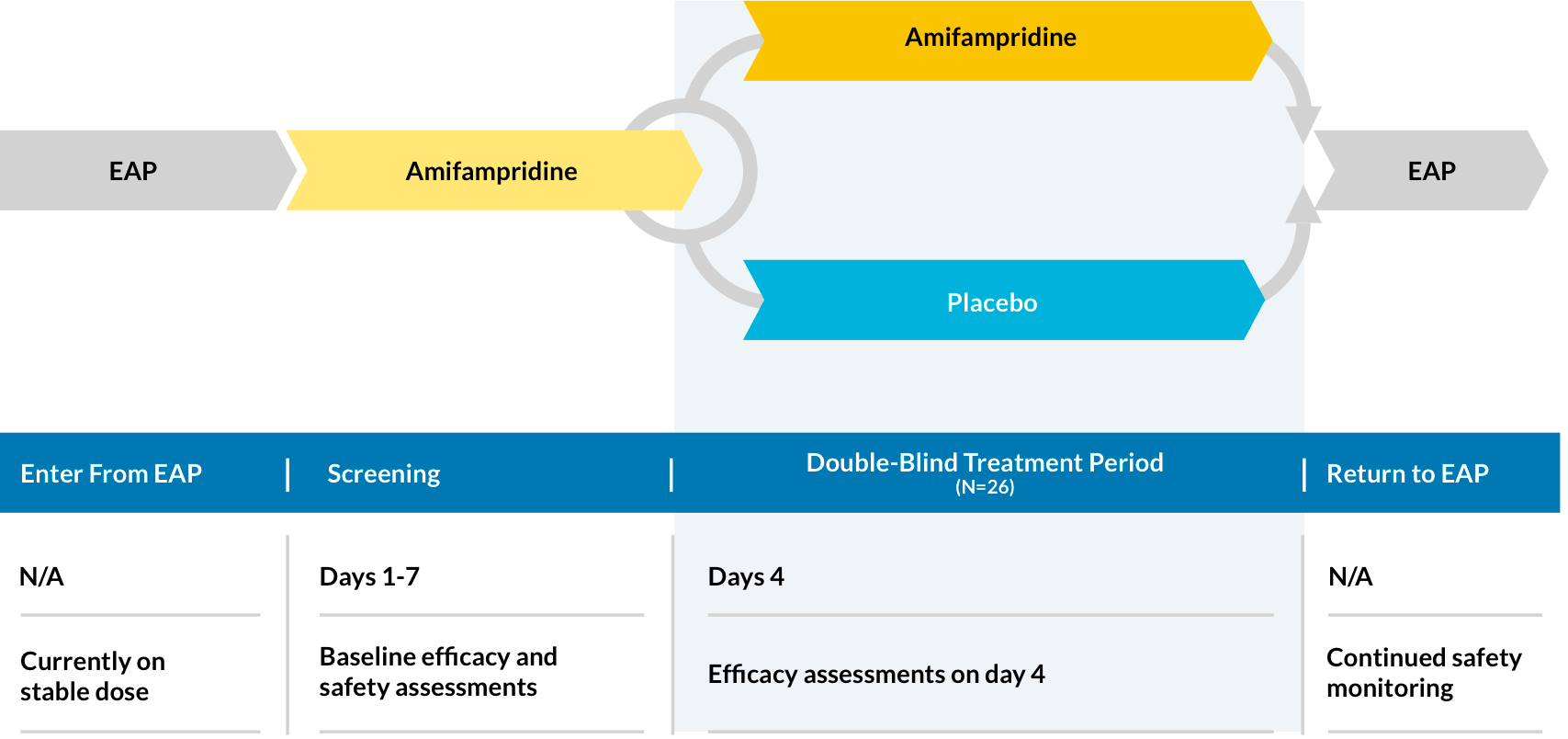
Second LEMS Phase 3 Trial (LMS-003)
LMS-003 is a phase 3, double-blind, placebo-controlled, randomized (1:1), parallel-group study to evaluate efficacy, safety, and the effect of amifampridine phosphate treatment in patients with LEMS.
Efficacy measures
Primary:
- Change in QMG score* from baseline (day 0) to end of day 4
- Change in SGI score† from baseline (day 0) to end of day 4
.
Secondary:
- Change in CGI-I score§ from baseline (day 0) to end of day 4
Exploratory:
- Change in Triple Timed Up and Go (3TUG) score from baseline (day 0) to complete test on day 4
Safety assessments
Patient safety is assessed during the screening period and throughout the EAP
CGI-I= Clinical Global Impression–Global Improvement; EAP=Expanded Access Program; NDA=new drug application; QMG= Quantitative Myasthenia Gravis; SGI=Subject Global Impression; T25FW=Timed 25-Foot Walk.
- The QMG is a physician-rated test using 13 assessments, including facial strength, swallowing, grip strength, and duration of time that limbs can be maintained in outstretched positions. Each of the 13 items is scored from 0 (none) to 3 (severe). The total score can range from 0 to 39. Increased QMG total score correlates to worsening symptoms of LEMS.
- The SGI is a measure of changes in a subject’s perception of change in overall well-being. The patient is asked to use the 7-point scale to rate their impression of the effects of the study medication, during the preceding 3 days, on their physical well-being. The 7-point SGI scale: 1=terrible; 2=mostly dissatisfied; 3=mixed; 4=partially satisfied; 5=mostly satisfied; 6=pleased; 7=delighted.
- The T25FW test, a component of the Multiple Sclerosis Functional Composite, was a quantitative mobility and leg function performance test based on a timed 25-foot walk (National Multiple Sclerosis Society). The patient was directed to walk a clearly marked 25-foot course as quickly and safely as possible. Following a rest of at least 5 minutes, the timed 25-foot walk was repeated. Patients could use assistive devices, such as canes, crutches, or walkers. (All data were normalized to the number of feet per minute, so if the patient walked 25 feet in less than a minute, the result was a speed greater than 25 feet/minute. The measurement for the T25FW test was the average speed, expressed in feet/minute, of the 2 completed walks.)
- The Investigator completed the 7-point CGI-I, based on changes in symptoms, behavior, and functional abilities, at the protocol-specified time points compared to the patient’s condition at day 0. The 7-point CGI-I scale: 1=very much improved; 2= much improved; 3=minimally improved; 4=no change; 5=minimally worse; 6=much worse; 7=very much worse.
- All randomized subjects and 2 subjects from part 1 (days 1-91) continued in the 2-year follow-up phase (N-40).
References:
- Oh Sj, Shcherbakova N, Kostera-Pruszczyk A, et al.; LEMS Study Group Amifampridine phosphate (Firdapse®) is effective and safe in a phase 3 clinical trial in LEMS. Muscle Nerve. 2016;53(5):717-725.
- Orphanet. Lambert-Eaton myasthenic syndrome. http://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=EN&data_id=10583&Disease_Disease_Search_diseaseGroup=Lambert-Eaton-myasthenic-syndrome&Disease_Disease_Search_diseaseType=Pat&Disease(s)/group%20of%20diseases=Lambert-Eaton-myasthenic-syndrome&title=Lambert-Eaton-myasthenic-syndrome&search=Disease_Search_Simple. Accessed December 13, 2017.
- Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol. 2011;10(12):1098-1107
- Harms l, Sieb JP, Williams AE et al. Long-term disease history, clinical symptoms, health status, and healthcare utilization in patients suffering from Lambert Eaton myasthenic syndrome: results of a patient interview survey in Germany. J Med Econ. 2012;15(3):521-530.
MuSK-MG Clinical
SMA Clinical
PIPELINE(CLINICAL)

Now there's an FDA-approved, first-line treatment for adults with LEMS.1,2
- A history of seizures
- Hypersensitivity to amifampridine phosphate or another aminopyridine