CORAL GABLES, Fla., December 13, 2018 – Catalyst Pharmaceuticals, Inc. (Catalyst) (Nasdaq: CPRX), a biopharmaceutical company focused on developing and commercializing innovative therapies for people with rare debilitating, chronic neuromuscular and neurological diseases, today held an investor call to discuss its plans to commercialize Firdapse® (amifampridine) 10 mg tablets, its product recently approved by the United States Food and Drug Administration for use in adults with Lambert-Eaton myasthenic syndrome.
A webcast replay is available if you click here.
For a transcript of the call, please click here.
About Catalyst Pharmaceuticals
Catalyst Pharmaceuticals is a biopharmaceutical company focused on developing and commercializing innovative therapies for people with rare debilitating, chronic neuromuscular and neurological diseases, including LEMS, congenital myasthenic syndromes (CMS), MuSK antibody positive myasthenia gravis (MuSK-MG), and spinal muscular atrophy (SMA) type 3. Amifampridine phosphate has received Orphan Drug Designation from the United States FDA for CMS and myasthenia gravis. Firdapse (amifampridine) 10 mg tablets is the first and only approved drug in Europe for symptomatic treatment in adults with LEMS.
Catalyst is also developing a generic version of vigabatrin.
About Firdapse® (amifampridine)
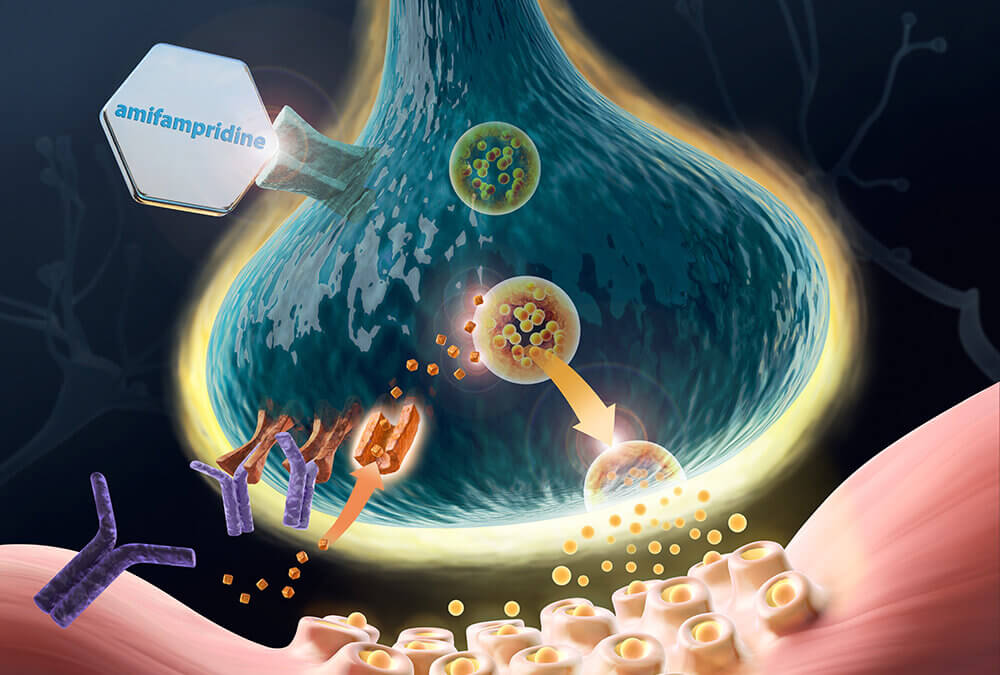
Firdapse® (amifampridine) 10 mg tablets is an oral, nonspecific, voltage-dependent, potassium (K+) channel blocker that causes depolarization of the presynaptic membrane and slows or inhibits repolarization. This action results in the opening of slow voltage-dependent calcium (Ca2+) channels, allowing for a subsequent influx of Ca2+. In turn, it induces the exocytosis of synaptic vesicles containing Acetylcholine (ACh) to release more ACh into the synaptic cleft, enhancing neuromuscular transmission, and providing for improved muscle function. Firdapse is approved in the U.S. and the European Union for use by patients with LEMS.
Important Safety Information
CONTRAINDICATIONS
FIRDAPSE is contraindicated in patients with:
- A history of seizures
- Hypersensitivity to amifampridine phosphate or another aminopyridine
WARNINGS AND PRECAUTIONS
Seizures: FIRDAPSE can cause seizures. Consider discontinuation or dose reduction of FIRDAPSE in patients who have a seizure while on treatment. FIRDAPSE is contraindicated in patients with a history of seizures.
Hypersensitivity: If a hypersensitivity reaction such as anaphylaxis occurs, FIRDAPSE should be discontinued and appropriate therapy initiated.
ADVERSE REACTIONS
The most common (> 10%) adverse reactions are: paresthesia, upper respiratory tract infection, abdominal pain, nausea, diarrhea, headache, elevated liver enzymes, back pain, hypertension, and muscle spasms.
To report SUSPECTED ADVERSE REACTIONS, contact Catalyst Pharmaceuticals at 1-844-347-3277 (1844-FIRDAPSE) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.